Drug development is an arduous and costly process, and failure rates in clinical trials that test new drugs for their safety and efficacy in humans remain high. According to current estimates, only 13.8 percent of all tested drugs demonstrate ultimate clinical success and obtain approval by the Food and Drug Administration (FDA). There are also increasing issues regarding animal studies, and a search for replacements.
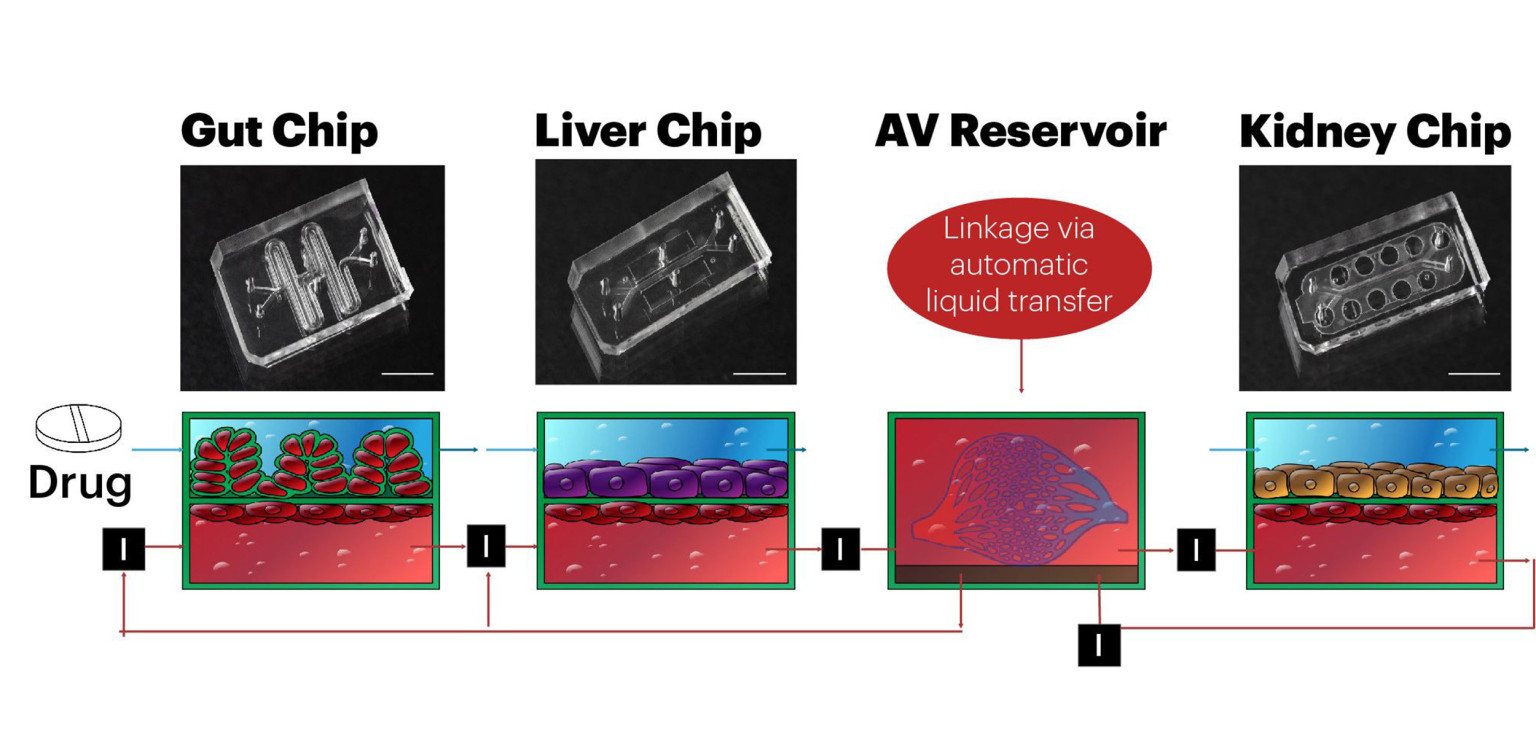
To help address this bottleneck in drug development, Donald Ingber and his team at Harvard’s Wyss Institute for Biologically Inspired Engineering, developed the first human “organ-on-a-chip” (organ chip) model of the lung that recapitulates human organ level physiology and pathophysiology with high fidelity, which was reported in Science in 2010. Organ chips are microfluidic culture devices composed of a clear flexible polymer the size of a computer memory stick, which contains two parallel hollow channels that are separated by a porous membrane. Organ-specific cells are cultured on one side of the membrane in one of the channels, and vascular endothelial cells recapitulating a blood vessel line the other, while each channel is independently perfused with cell type-specific medium. The porous membrane allows the two compartments to communicate with each other, and to exchange molecules like cytokines, growth factors, and drugs, as well as drug breakdown products generated by organ-specific metabolic activities.
One example where animals are needed in preclinical testing is the characterization of a drug’s “pharmacokinetics” (PK) that involves the quantification of its absorption, distribution, metabolism, and excretion (ADME), which together determine drug levels in the blood. These responses involve interplay between different organs linked by a vasculature containing flowing blood.
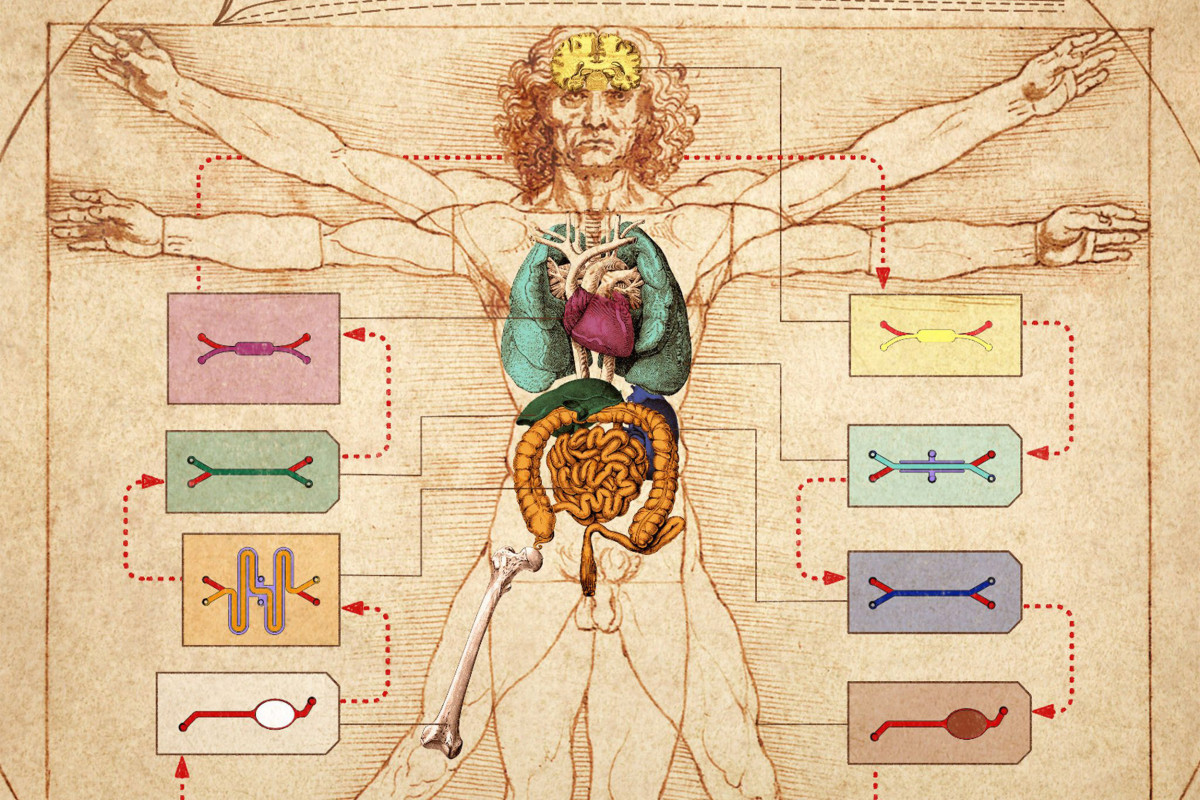
Because the organ chips contain an endothelium-lined vascular channel, Ingber proposed in 2011 that it might be possible to create a human “body-on-chips” by transferring fluids between the vascular channels of many different types of organ chips to mimic blood flow, and assessing drug PK/PD behaviors across the entire linked system. Inspired by this vision and the realization that existing animal-based development programs are inadequate to confront the needs for accelerated development of drug countermeasures in a biothreat situation, the Defense Advanced Research Projects Agency (DARPA) requested grant applications in 2012 with a seemingly impossible challenge: develop 10 types of organ chips that recapitulate the complex functionalities of 10 different human organs, engineer an automated instrument to fluidically link them to create a functional human body-on-chips platform, and leverage computational modeling in combination with experimental data generated using this platform to quantitatively predict human drug PK/PD behavior in vitro.
Now, two back-to-back publications in Nature Biomedical Engineering, describe the Wyss team’s success in meeting this goal in full.
Known for posing impossible challenges such as this, DARPA understands that most investigators will not meet the goals as set out, but that extraordinary technological fallout will be created along the way. “We were very proud to obtain major funding support from DARPA to take on this challenge, and we are now even more proud that we have successfully met their goal, which would not have been possible without the exceptional talents, interdisciplinary spirit, and monumental team effort at the Wyss Institute,” said Ingber, who is the Wyss founding director, as well as the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital, and professor of bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS). He has been leading the DARPA-funded program along with Wyss Core Faculty member Kevin Kit Parker, who also is the Tarr Family Professor of Bioengineering and Applied Physics at SEAS.
In their first article, the Wyss team presents a highly modular body-on-chips platform, which is enabled by an engineered “Interrogator” instrument that can culture up to 10 different organ chips, and sequentially transfer fluids between their endothelium-lined vascular channels to mimic normal human blood flow between the different organs of our body. In the second article, the team uses a computational scaling method to translate data obtained from drug experiments involving three different types of fluidically linked organ chips to their respective organ dimensions in the real human body. The approach is able to quantitatively predict changes in drug levels over time, as well as organ-specific toxicities, that have been previously measured in human patients.
“Both studies represent a tremendous effort by scores of researchers at the Wyss Institute, who worked together with our industrial modeling collaborators, and pooled their collective tissue engineering, microfabrication, pharmacological, physiological, and computational expertise to make this huge advance in preclinical drug testing possible,” said Rachelle Prantil-Baun, a Wyss Institute senior staff scientist with past experience in the pharmaceutical industry who helped to orchestrate this complex multi-investigator effort with multiple other staff members in the Wyss’ Bioinspired Therapeutics and Diagnostics platform.
The Interrogator instrument enabled the team to culture, perfuse and link many living human cultured tissues in a multi-organ chip system, as well as add and sample the medium in a fully programmable way, using the device’s robotic liquid transfer capabilities, while continuously monitoring tissue integrity with an integrated microscope. “In this study, we serially linked the vascular channels of eight different organ chips, including intestine, liver, kidney, heart, lung, skin, blood–brain barrier and brain, using a highly optimized common blood substitute, while independently perfusing the individual channels lined by organ-specific cells. The instrument maintained the viability of all tissues and their organ-specific functions for over three weeks and, importantly, it allowed us to quantitatively predict the tissue-specific distribution of a chemical across the entire system,” said Richard Novak, a co-first-author on both studies. Novak is a Senior Staff Engineer at the Wyss Institute who designed, fabricated, and operated the Interrogator instrument with his bioengineering team.
In their first article, the Wyss team presents a highly modular body-on-chips platform, which is enabled by an engineered “Interrogator” instrument that can culture up to 10 different organ chips, and sequentially transfer fluids between their endothelium-lined vascular channels to mimic normal human blood flow between the different organs of our body. In the second article, the team uses a computational scaling method to translate data obtained from drug experiments involving three different types of fluidically linked organ chips to their respective organ dimensions in the real human body. The approach is able to quantitatively predict changes in drug levels over time, as well as organ-specific toxicities, that have been previously measured in human patients.
“Both studies represent a tremendous effort by scores of researchers at the Wyss Institute, who worked together with our industrial modeling collaborators, and pooled their collective tissue engineering, microfabrication, pharmacological, physiological, and computational expertise to make this huge advance in preclinical drug testing possible,” said Rachelle Prantil-Baun, a Wyss Institute senior staff scientist with past experience in the pharmaceutical industry who helped to orchestrate this complex multi-investigator effort with multiple other staff members in the Wyss’ Bioinspired Therapeutics and Diagnostics platform.
The Interrogator instrument enabled the team to culture, perfuse and link many living human cultured tissues in a multi-organ chip system, as well as add and sample the medium in a fully programmable way, using the device’s robotic liquid transfer capabilities, while continuously monitoring tissue integrity with an integrated microscope. “In this study, we serially linked the vascular channels of eight different organ chips, including intestine, liver, kidney, heart, lung, skin, blood–brain barrier and brain, using a highly optimized common blood substitute, while independently perfusing the individual channels lined by organ-specific cells. The instrument maintained the viability of all tissues and their organ-specific functions for over three weeks and, importantly, it allowed us to quantitatively predict the tissue-specific distribution of a chemical across the entire system,” said Richard Novak, a co-first-author on both studies. Novak is a Senior Staff Engineer at the Wyss Institute who designed, fabricated, and operated the Interrogator instrument with his bioengineering team.
| Science Abstract: |
|
Reconstituting Organ-Level Lung Functions on a Chip
|
| Here, we describe a biomimetic microsystem that reconstitutes the critical functional alveolar-capillary interface of the human lung. This bioinspired microdevice reproduces complex integrated organ-level responses to bacteria and inflammatory cytokines introduced into the alveolar space. In nanotoxicology studies, this lung mimic revealed that cyclic mechanical strain accentuates toxic and inflammatory responses of the lung to silica nanoparticles. Mechanical strain also enhances epithelial and endothelial uptake of nanoparticulates and stimulates their transport into the underlying microvascular channel. Similar effects of physiological breathing on nanoparticle absorption are observed in whole mouse lung. Mechanically active “organ-on-a-chip” microdevices that reconstitute tissue-tissue interfaces critical to organ function may therefore expand the capabilities of cell culture models and provide low-cost alternatives to animal and clinical studies for drug screening and toxicology applications. |
Reference:
- https://science.sciencemag.org/content/328/5986/1662.long
- https://news.harvard.edu/gazette/story/2020/01/human-body-on-chip-platform-may-speed-up-drug-development/
- https://player.vimeo.com/video/380062652