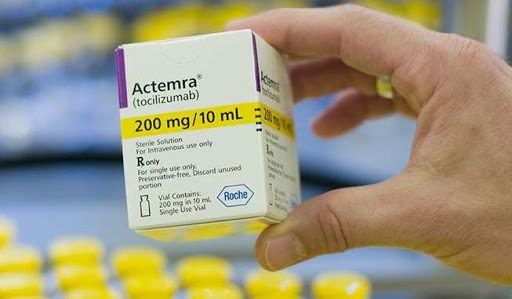
A drug used to treat inflammation in arthritis patients, Actemra, could be the answer to coronavirus cure.
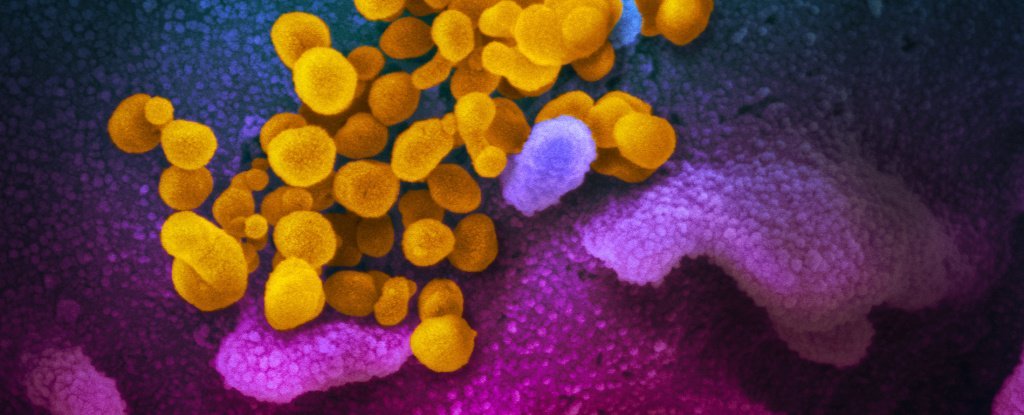 |
| COVID-19 virus scanning and transmission electron microscope image released by the National Institute of Allergy and Infectious Diseases’ (NIAID) Rocky Mountains Laboratories (RML) Ref. |
Swiss drugmaker Roche on Wednesday got China’s approval for the anti-inflammation drug Actemra that gave hope to fight the deadly coronavirus in which over 95,000 people have been infected and over 3,200 have died worldwide.
The latest move comes as China’s National Health Commission is searching for new ways to combat the deadly virus, which is spreading to other countries, including the Italy, South Korea, Iran, France, Spain, US, and Japan.
In its latest treatment guidelines published online, the commission said that the biologic drug Tocilizumab, sold by the Swiss pharma giant Roche under the trade name Actemra, can now be used to treat coronavirus patients with severe lung damage and high Interleukin 6 (IL-6) levels.
In 2010, Actemra secured approval from the US Food and Drug Administration (FDA) for the treatment of rheumatoid arthritis (RA). The drug is capable of inhibiting high IL-6 protein levels which could indicate inflammation or immunological diseases.
Chinese researchers recently registered a 3-month clinical trial for Actemra that will recruit 188 coronavirus patients and take place from Feb. 10 to May 10, according to records shown on China’s clinical trials registration database.
Roche was quoted by Reuters as saying that a third party has initiated the trial independently to explore the efficacy and safety of the drug in coronavirus patients with cytokine release syndrome (CRS). At present, no published clinical trial data is available on the safety or efficacy of Actemra against the Wuhan virus, which has claimed the lives of more than 3,200 people as of the end of 4 March.
Other drug manufacturers in the country are also in the race to develop alternatives to Roche’s treatment. Bio-Thera Solutions expects to file new drug approval for its Actemra biosimilar in 2021, and Zhejiang Hisun Pharmaceutical received in 2016 regulatory approval to conduct clinical trials for its Tocilizumab candidate, company filings showed. Biosimilars are cheaper versions of complex biotech drugs such as Actemra.
Reference:
- https://www.pharmaceutical-technology.com/news/roche-actemra-coronavirus-complications/
- https://www.channelnewsasia.com/news/business/china-approves-use-of-roche-arthritis-drug-for-covid-19-patients-12499492
- https://www.deccanherald.com/science-and-environment/actemra-the-drug-that-could-be-the-cure-for-the-novel-coronavirus-810817.html