Roche unit Genentech has received US Food and Drug Administration (FDA) approval to conduct a Phase III clinical trial of its rheumatoid arthritis drug Actemra (tocilizumab) for the treatment of adults with severe Covid-19 pneumonia.
To be performed in partnership with the Biomedical Advanced Research and Development Authority (BARDA), the trial will assess a combination of intravenous Actemra and standard of care in hospitalised patients. Genentech also agreed to supply 10,000 vials of the drug to the US Strategic National Stockpile for potential future use at the US Department of Health and Human Services (HHS)’s direction. The company noted that supply of the drug for approved indications in the US is not expected to be affected. It is also collaborating with distributors to manage product supply to cater to patient requirements.
Genentech CEO Alexander Hardy said: “We thank the FDA for rapidly expediting the approval of this clinical trial to evaluate Actemra in critically ill patients suffering from pneumonia following the coronavirus infection and we’re moving forward to enroll as quickly as possible.“Conducting this clinical trial in partnership with BARDA and providing Actemra to support the national stockpile, through the efforts of Secretary Azar and HHS, are important examples of how the US Government, the biotechnology industry, and healthcare communities are working together in response to this public health crisis.”
Multiple independent studies are currently being conducted worldwide to assess Actemra in patients with Covid-19 pneumonia. Roche announced plans to conduct the Phase III trial in the US earlier this month. The randomised, double-blind, placebo-controlled COVACTA study is designed to compare the safety and efficacy of Actemra plus standard of care to placebo plus standard of care. Patients will be followed for 60 days following randomisation. Interim analysis will be performed to analyse early data of efficacy.
About Actemra
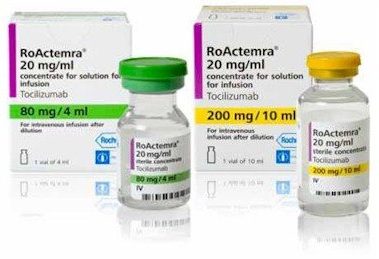 Actemra was the first humanized interleukin-6 (IL-6) receptor antagonist approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis (RA) who have used one or more disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX), that did not provide enough relief. The extensive Actemra RA IV clinical development program included five Phase III clinical studies and enrolled more than 4,000 people with RA in 41 countries. The Actemra RA subcutaneous clinical development program included two Phase III clinical studies and enrolled more than 1,800 people with RA in 33 countries. Actemra subcutaneous injection is also approved for the treatment of adult patients with giant cell arteritis (GCA) and for patients two years of age and older with active polyarticular juvenile idiopathic arthritis (PJIA) or active systemic juvenile idiopathic arthritis (SJIA). In addition, Actemra is also approved in the IV formulation for patients two years of age and older with active PJIA, SJIA or CAR T cell-induced cytokine release syndrome (CRS). Actemra is not approved for subcutaneous use in people with CRS. It is not known if Actemra is safe and effective in children with PJIA, SJIA or CRS under two years of age or in children with conditions other than PJIA, SJIA or CRS.
Actemra was the first humanized interleukin-6 (IL-6) receptor antagonist approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis (RA) who have used one or more disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX), that did not provide enough relief. The extensive Actemra RA IV clinical development program included five Phase III clinical studies and enrolled more than 4,000 people with RA in 41 countries. The Actemra RA subcutaneous clinical development program included two Phase III clinical studies and enrolled more than 1,800 people with RA in 33 countries. Actemra subcutaneous injection is also approved for the treatment of adult patients with giant cell arteritis (GCA) and for patients two years of age and older with active polyarticular juvenile idiopathic arthritis (PJIA) or active systemic juvenile idiopathic arthritis (SJIA). In addition, Actemra is also approved in the IV formulation for patients two years of age and older with active PJIA, SJIA or CAR T cell-induced cytokine release syndrome (CRS). Actemra is not approved for subcutaneous use in people with CRS. It is not known if Actemra is safe and effective in children with PJIA, SJIA or CRS under two years of age or in children with conditions other than PJIA, SJIA or CRS.
 |
| Genentech, Inc., is a biotechnology corporation which became a subsidiary of Roche in 2009. Genentech Research and Early Development operates as an independent center within Roche. |
Reference:
- https://www.pharmaceutical-technology.com/news/genentech-fda-actemra-covid-19-trial/
- https://www.gene.com/media/press-releases/14843/2020-03-23/genentech-announces-fda-approval-of-clin